Quality Management
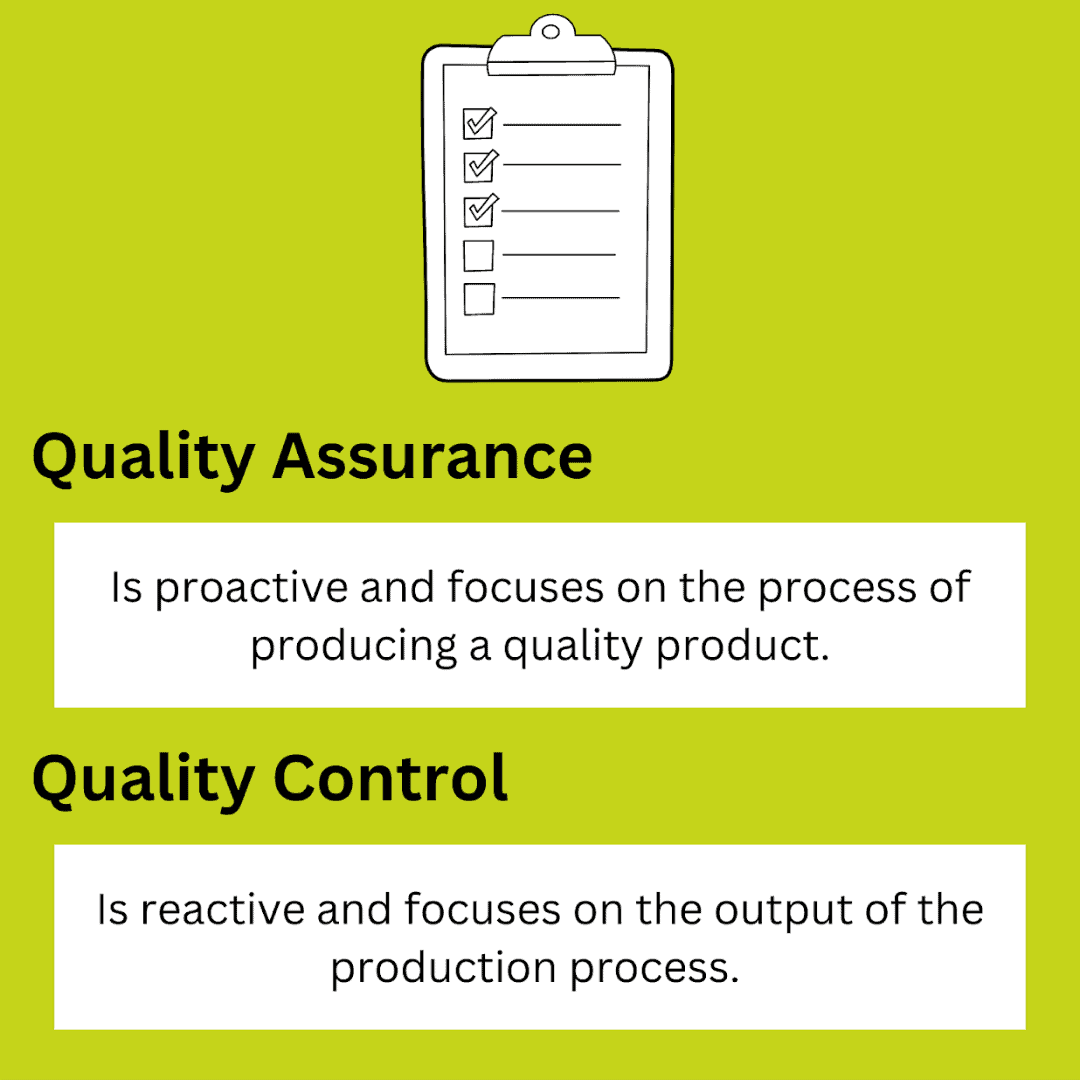
Quality management is broken down into two processes, quality assurance and quality control, with the former being the processes used to ensure a quality output
All too often, our nascent industry uses a “squeeze the bud, sniff the bag” technique to determine quality. All too often, this is inconsistent and non-replicable by its subjective nature – which makes discerning better prices for better quality just as commoditized and generalized.
Standardization
There are no officially adopted marijuana standards, although there are organizations that offer standardization certification. It is really up to companies to develop standards on their own. It may be years before a quality standard is universally accepted or third-party measures are developed and validated.
All licensed marijuana businesses are required to develop, implement, and manage standard operating procedures (SOPs). SOPs are critical as literal step-by-step procedures wherein a company develops a consistent product and defines its quality management system.
SOPs are written instructions that are easily trainable. Well developed SOPs are a part of the quality assurance process and assist in assessing the quality of the assurance processes used and where improvements need to be made.
Internal Controls and Testing
Many successful marijuana manufacturers implement internal controls and testing methods to ensure quality along the production line. Marijuana concentrates manufacturers, for example, will purchase testing equipment to test products during stages of development for internal quality control to determine consistent potency. Shelf stability testing, sampling managers, ingredients, and product formulation all use internal controls and testing to determine stability and expiration dates, for example.
Documentation and record keeping are essential steps to maintain during the production process to monitor and improve quality control measures and are required for every production batch in most states. States require process documentation to limit product liability and to determine how and where quality deviates as it concerns public safety in addition to standard operating procedures. This is helpful for an operation should a product or production process fail quality control measures or otherwise be subject to a Corrective Action Preventative Action Plan or, God forbid, a recall.
External Testing
Testing is the most critical step in quality assurance and compliance. All licensed cannabis businesses are required to test final products, whether it’s flower, prerolls, concentrates, edibles, or alternative use products like metered inhalers. Depending on the product, test results may vary.
External testing is also used to achieve process validation, also known in some states as “reduced testing allowance,” which happens when a product reaches consistent results using the same process – which does not deviate. When a product achieves process validation, companies can forgo some of the required testing steps and test less often, saving them time and money and increasing profitability.
Unfortunately, testing has been coming under fire lately across the United States, with many labs being accused of faking testing results to show higher THC levels or even scarier, to hide contamination concerns. All of this has led to businesses shopping for testing labs, and even worse, consumer confidence in licensed businesses is being impacted. This is yet another reason to have a solid quality assurance program internally, as it is critical to consistently creating a high quality product – which creates brands and product lines in higher demand, which can demand higher prices. Generic Cola is never going to be Coca-Cola for this reason.
Good Practices
Fortunately, there is no need to reinvent the wheel to develop a reliable quality management program simply because you’re working in the cannabis space. Federal regulations governing other industries have already developed universal practices to ensure quality which may be adapted to cannabis with some expertise. It is from these regulations that cannabis regulations are drawn from, so implementing them in your operating procedures will help ensure quality control.
Cultivation
The cultivation of marijuana should always use Good Agricultural Practices (GAP). GAP is a voluntary certification process provided by the United States Department of Agriculture (USDA). It is based on a set of standards that help producers grow high quality high, yielding produce that consumers can trust. Businesses can use their standards as metrics for measuring their own internal quality management program.
Processing
Processing cannabis for concentrates, edibles, topicals, capsules, and pre- rolls must follow Current Good Manufacturing Practices (cGMPs). Known as the Title 21 of the CFR (Code for Federal Rules), these rules set safety and sanitary standards for the production of foods which are also used for processing other products. HAACP controls are also being required in more and more States and should be ingrained into SOPs and be a part of QA/QC programs.
Responsible Training Programs
Quality and safety also guide dispensary and delivery operations. State regulations also govern sanitary and secure work environments for dispensing, hospitality, and delivery services, and the majority of states require vendor training. While responsible vendor training exists to assist in understanding rules and regulations for those who sell marijuana to consumers, we understand that compliance is often more complicated on the production side of the supply chain.
As such, we suggest that operators consider Responsible Cultivation and Manufacturing programs offered by companies like ours that help employees across the organizational chart to understand the consequences of non-compliance, contamination, and mismanagement of quality. These expensive, real-world examples and consequences are the foundation of good compliance and quality management which takes a team to conduct consistently and accurately.
Develop Good Habits
Overall, it is best to develop good habits from the beginning, and always more expensive and difficult to re-train and re-create a culture that has only known bad habits. Cleanliness and keeping things organized and documented is a simple daily practice to ensure quality.
Developing complex quality management systems and passing mandated products testing is key- but so is testing simple systems daily. Equipment and surfaces can be swabbed by inspectors, so it’s just best to be sure your facilities are always clean. Equipment and surfaces should be wiped down daily with a solution of bleach and water or isopropyl rubbing alcohol – especially after each batch. Keep in mind that there are also airborne particulates that can contaminate surfaces – including drifting pesticides from neighbors.
Try not to buy used equipment which may have been used with prohibited chemicals and if you have to, be sure to sanitize every surface and all of the parts of the equipment. Every piece of equipment and the facility itself should have a maintenance SOP in addition to sanitization to ensure operability and to reduce downtime risk.
Implement a quality management program while developing your SOPs. This way, should production not meet the standards in your SOPs, it is easier to determine which steps need amending to make improvements in quality. Frequently test and calibrate equipment as needed to ensure accuracy for internal controls. SOPs are never a one-time checkbox for compliance, they are a living document, your company’s intellectual property, and the guidebook for operational compliance and quality. They should be continually updated and maintained.
By developing good habits early on, you’re creating a brand known for quality that will withstand growth pains and overcome competition to keep regulators off your back and to leave your competition in the dust.
How iComply Can Help
iComply has the compliance leadership and practical operational compliance experience to help create a stellar quality management program as well as assist in providing operational analysis to discover any compliance gaps and inefficiencies.
Developing & implementing a quality management program first starts with creating SOPs and then monitoring and testing the production process to ensure operational integrity and highest quality results. With over a decade of experience in helping businesses develop quality management and compliance programs for the entire cannabis production life cycle.
We offer a variety of compliance packages and services that can meet your needs in developing and implementing best practices. We can also train your staff and are certified in many states to provide responsible training programs.
There are a million ways to do things wrong in the cannabis industry, but only a few ways of doing things right. Contact us today and save up to 30% off of our compliance packages and services.
